LRRK2 & Parkinson's Disease
Parkinson's disease (PD) is the most prevalent motor neurodegenerative disorder, primarily affecting older adults. It is characterized by the progressive degeneration of dopaminergic neurons, leading to a wide range of symptoms, with motor impairments being the most prominent, alongside non-motor complications. As the global population ages, neurodegenerative diseases like PD place an increasing burden on society.
While current treatments focus on alleviating symptoms and improving patients' quality of life, they do not stop disease progression. The main therapeutic approach aims to increase dopamine levels to compensate for the loss of dopaminergic neurons, while other treatments, including surgical interventions, help to control tremors. However, there is an urgent need for the discovery of new therapeutic targets to more effectively combat PD.
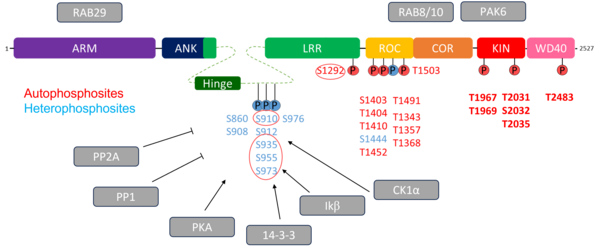
Leucine-rich repeat kinase 2 (LRRK2) has emerged in recent decades as a key therapeutic target for PD. LRRK2 is a large protein, comprising over 2,500 amino acids, structured into seven functional domains: three that form its enzymatic core (Roc, COR, and Kinase) and four domains featuring repeated motifs that mediate protein-protein interactions (ARM, ANK, LRR, and WD40). LRRK2 plays a role in numerous cellular processes and is tightly regulated, both in its normal and pathological functions, through multiple phosphorylation sites. Several pathogenic mutations in LRRK2 have been identified in PD patients. Within our team, we are pursuing multiple strategies to target LRRK2 in the fight against PD:
- Investigating the interaction between LRRK2 and the other key pathological protein in PD, alpha-synuclein, through a third partner: VAMP proteins. This interaction plays a role in the regulation of cellular trafficking and protein homeostasis, two processes that are disrupted in the disease.
- Modulating the phosphorylation of LRRK2. Certain specific phosphorylation sites (heterologous) need to remain phosphorylated to offer protection or slow disease progression. We are exploring ways to stabilize the interaction between LRRK2 and 14-3-3 proteins, which safeguard these phosphorylated sites, as well as approaches to inhibit the interaction between LRRK2 and phosphatases that dephosphorylate these protective sites.
- Targeting other protein-protein interactions involving LRRK2. LRRK2 is potentially involved with hundreds of proteins, and we are evaluating the therapeutic potential of these interactions as new treatment targets for PD.