Item 7 - New therapeutic strategies thanks to pharmacological cerebral modulation
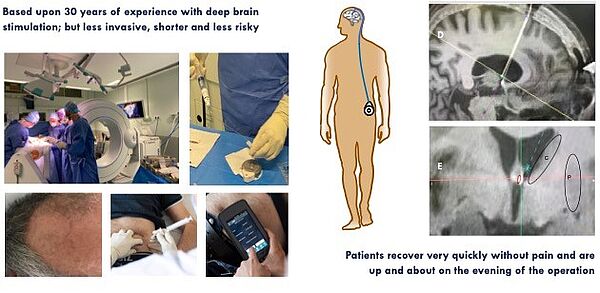
Cerebral pharmacological neuromodulation by anaerobic dopamine for Parkinson's disease: DIVE-I study
Parkinson's disease is characterized by nigrostriatal dopamine depletion. The main symptomatic oral treatment for PD is L-dopa, the precursor of dopamine, because dopamine does not cross the digestive and blood-brain barriers. Due to numerous pharmacokinetic and pharmacodynamic biases, L-dopa is the cause of very disabling motor complications (on/off periods, dyskinesias) in 50% of Parkinson's patients after 5 years, and 80% after 10 years.
For more than 50 years, no one had developed the intracerebral administration of dopamine to combat these complications linked to L-dopa, due to the fear of dopamine oxidation. We overcame this barrier by preparing and administering dopamine anaerobically (Laloux et al., 2017; Moreau et al., 2020). With this patent (EP15305352.5), we have validated the very high effectiveness in mouse, rat and monkey models of Parkinson's disease.
We have just completed a Phase I/II therapeutic trial demonstrating very high efficacy in Parkinson's patients. A very large Phase III trial allowing the registration of this new therapeutic strategy is currently in preparation with the start-up InBrain Pharma and the support of our entire university hospital campus.
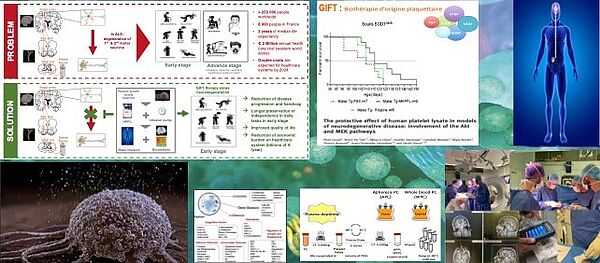
Innovative biotherapy using the platelet repair system to treat ALS: RHU: SECRET-GIFT
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of motor neurons, leading to progressive paralysis and irreversible death within a variable time frame, with a median of three years. Currently, there is no cure and no satisfactory improvement in life expectancy. It is therefore urgent to develop an innovative and effective therapeutic strategy, taking into account the multiplicity of mechanisms involved in the disease.
Over the last 10 years, we have developed and patented a biotherapy based on human platelets, presenting all the safety requirements for intracerebral administration, and which has demonstrated its immense neuroprotective activity in a large number of experimental models.
This advance was made possible thanks to collaborations with the French Blood Establishments, internationally recognized specialists in platelet concentrates and neurodegenerative diseases, and the start-up InVenis Biotherapeutics.
With SECRET-GIFT, the winning project of wave 6 of the RHU, led by the University of Lille in collaboration with the Lille University Hospital, we aim to demonstrate the feasibility, safety and effectiveness of our innovative biotherapy in ALS patients. , using continuous methods, safe intracerebral administration.
This project, which brings together the best experts in the field, is structured around three axes: development of biomarkers to assess the severity of the disease; industrial production of the treatment in accordance with the regulations in force for a clinical trial; implementation of a first therapeutic trial to validate the safety and effectiveness of the treatment. We hope to bring a major improvement to the life expectancy and quality of life of people with ALS, and thus bring therapeutic hope to a fatal and currently incurable disease.