Item 5 - Transforming the prognosis of intracerebral hemorrhage
In 2024, strokes will be the leading cause of physical disability among adults worldwide. Among the different subtypes of stroke, spontaneous intracerebral hemorrhage (ICH) is the second most common subtype, affecting 3.5 million people worldwide each year.
ICH is the most fatal form of acute stroke, with an early mortality of approximately 40% and no or minimal tendency for improvement over time. Among the survivors, half are dependent. The PCI therefore has enormous social and economic impacts. The lack of effective treatment at present is responsible for this medico-social tragedy. A single intervention is unlikely to resolve the prognosis challenge of ICH.
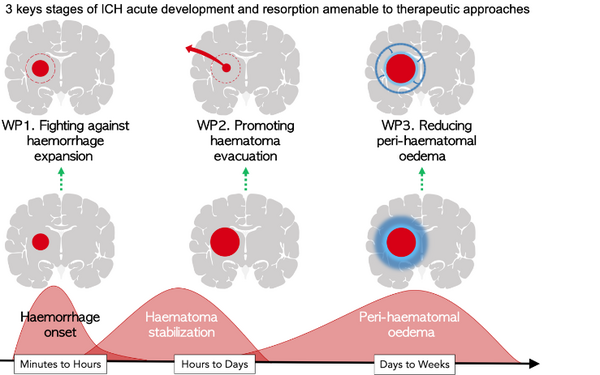
Indeed, there are three key stages in the pathophysiology of ICH occurring at successive times that should be targeted by complementary innovative therapeutic approaches. TIPITCH will develop these complementary innovative therapeutic approaches by focusing on:
- Fight against the expansion of hemorrhages. With our industrial partner BALT, we will develop an endovascular device to locally modulate intracranial blood flow. From proof of concept in animal models to validation of clinical trials, this will lead to a newly designed device which will be subject to CE marking.
- Promote the evacuation of hematomas. We will focus on patients who were excluded from clinical studies due to their high bleeding risk. In collaboration with our industrial partner Op2Lysis, we will evaluate the safety and effectiveness of a modified form of rtPA, on innovative and dedicated ex vivo models of hematomas imitating situations with high hemorrhagic risk. At the end of TIPITCH, we will design a randomized controlled trial (RCT) of minimally invasive surgery, dedicated to patients at high risk of bleeding complications, using the modified form of rtPA (O2L-001).
- Promotes the evacuation of hematomas. We will focus on patients who were excluded from clinical studies due to their high bleeding risk. In collaboration with our industrial partner Op2Lysis, we will evaluate the safety and effectiveness of a modified form of rtPA, on innovative and dedicated ex vivo models of hematomas imitating situations with high hemorrhagic risk. At the end of TIPITCH, we will design a randomized controlled trial (RCT) of minimally invasive surgery, dedicated to patients at high risk of bleeding complications, using the modified form of rtPA (O2L-001).
- Structure a national network dedicated to ICH research and care. We will organize the national TIPITCH network and gradually implement the results of TIPITCH in addition to other cutting-edge treatments. We will develop a multimodal observational ICH registry (with creation of a clinical, radiological and biological database) in collaboration with the French Clinical Research Infrastructure Networks (FCRIN) StrokeLink and Tech4health. Patient- and family-centered measures, as well as communication methods that optimize patient- and family-centered outcomes, will be integrated into our program.